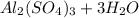Chemistry, 11.01.2021 22:30 JessTaylr04
For the following reaction, 3.04 grams of sulfuric acid are mixed with excess aluminum oxide. The reaction yields 2.53 grams of aluminum sulfate. aluminum oxide (s) sulfuric acid (aq) aluminum sulfate (aq) water (l) What is the theoretical yield of aluminum sulfate

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

You know the right answer?
For the following reaction, 3.04 grams of sulfuric acid are mixed with excess aluminum oxide. The re...
Questions

Mathematics, 21.04.2021 23:50

Spanish, 21.04.2021 23:50




Mathematics, 21.04.2021 23:50


Physics, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50


History, 21.04.2021 23:50


Chemistry, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50



Mathematics, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50

Chemistry, 21.04.2021 23:50

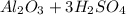 →
→