
Chemistry, 12.01.2021 01:00 aaronhorowitz36
4.92x1022 atoms Na = mol Na2O Please let me know how you solved it also, requires work to be shown

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
4.92x1022 atoms Na = mol Na2O
Please let me know how you solved it also, requires work to be shown...
Questions

Biology, 18.12.2019 21:31

English, 18.12.2019 21:31

English, 18.12.2019 21:31

History, 18.12.2019 21:31
















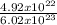 = 0.82 x 10⁻¹ = 0.082mole
= 0.82 x 10⁻¹ = 0.082mole 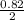 mole of Na₂O = 0.41mole of Na₂O
mole of Na₂O = 0.41mole of Na₂O

