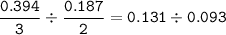Chemistry, 12.01.2021 14:00 24wilsleaann
If you react 25.0g of Cu with 25.0g of AlCl3 in the following reaction 3Cy + 2AlCl3 -> 3CuCl2 + 2Al
a. find the excess and limiting reactants
b. calculate the mass of leftover reactant

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
If you react 25.0g of Cu with 25.0g of AlCl3 in the following reaction 3Cy + 2AlCl3 -> 3CuCl2 + 2...
Questions

Mathematics, 17.07.2019 17:30

Mathematics, 17.07.2019 17:30







English, 17.07.2019 17:30

Biology, 17.07.2019 17:30

Computers and Technology, 17.07.2019 17:30

Arts, 17.07.2019 17:30