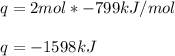Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1


Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
When 2 moles of Fe(s) react with Cl2(g) to form FeCl3(s) according to the following equation, 799 kJ...
Questions


Social Studies, 11.02.2021 02:50



History, 11.02.2021 02:50

Business, 11.02.2021 02:50


English, 11.02.2021 02:50

Business, 11.02.2021 02:50



Mathematics, 11.02.2021 02:50

Mathematics, 11.02.2021 02:50

Mathematics, 11.02.2021 02:50

Chemistry, 11.02.2021 02:50


Mathematics, 11.02.2021 02:50

Mathematics, 11.02.2021 02:50

Mathematics, 11.02.2021 02:50

Chemistry, 11.02.2021 02:50