
Chemistry, 12.01.2021 17:50 alexandrarosete7
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 22 atm at an absolute temperature of 353K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other than R.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions

Mathematics, 29.03.2020 08:12

Mathematics, 29.03.2020 08:12



Mathematics, 29.03.2020 08:12



Mathematics, 29.03.2020 08:13



Mathematics, 29.03.2020 08:13

Mathematics, 29.03.2020 08:13

Mathematics, 29.03.2020 08:13


Chemistry, 29.03.2020 08:13

Mathematics, 29.03.2020 08:13


Mathematics, 29.03.2020 08:14

Health, 29.03.2020 08:14

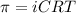
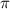 = osmotic pressure exerted by a solution
= osmotic pressure exerted by a solution

