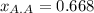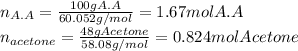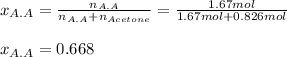Chemistry, 12.01.2021 18:30 hannahliebl2000
A solution is made by mixing 100.g of acetic acid HCH3CO2 and 48.g of acetone CH32CO. Calculate the mole fraction of acetic acid in this solution. Round your answer to 3 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
A solution is made by mixing 100.g of acetic acid HCH3CO2 and 48.g of acetone CH32CO. Calculate the...
Questions




History, 02.10.2019 22:40




History, 02.10.2019 22:40

Biology, 02.10.2019 22:40






Mathematics, 02.10.2019 22:40




History, 02.10.2019 22:40