
Chemistry, 13.01.2021 15:40 olivya2005d
If you had excess chlorine, how many moles of of aluminum chloride could be produced from 11.0 g of aluminum

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
If you had excess chlorine, how many moles of of aluminum chloride could be produced from 11.0 g of...
Questions

Mathematics, 05.07.2020 22:01

Mathematics, 05.07.2020 22:01

Chemistry, 05.07.2020 22:01





Mathematics, 05.07.2020 22:01



Advanced Placement (AP), 05.07.2020 22:01

Mathematics, 05.07.2020 22:01

Mathematics, 05.07.2020 22:01




Mathematics, 05.07.2020 22:01

Mathematics, 05.07.2020 22:01

Mathematics, 05.07.2020 22:01

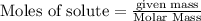
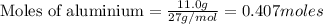
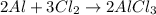
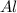 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and 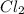 is the excess reagent.
is the excess reagent.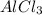
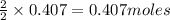 of
of 


