
Chemistry, 13.01.2021 19:00 marygatewell385
HELP ASAP
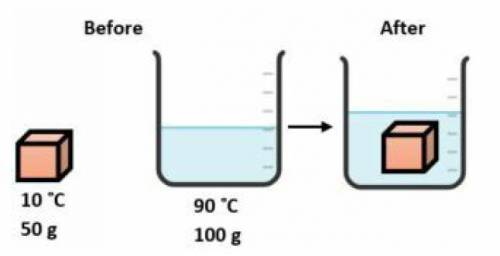
A 50.0 gram block of copper at 10.0 °C is carefully lowered into 100.0 grams of water at 90.0 °C in an insulated container as shown below. Which statement describes the transfer of heat in this system?
The water loses heat and the block gains heat until both are at the same temperature that is between 10.0°C and 90.0°C.
The water loses heat and the block gains heat until both are at the same temperature that is between 10.0°C and 90.0°C.
The water gains heat and the block loses heat until both are at the same temperature that is between 10.0°C and 90.0°C.
The water gains heat and the block loses heat until both are at the same temperature that is between 10.0°C and 90.0°C.
The water loses heat to the block until both are at 10.0°C.
The water loses heat to the block until both are at 10.0°C.
The block gains heat from the water until both are at 90.0°C.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
HELP ASAP
A 50.0 gram block of copper at 10.0 °C is carefully lowered into 100.0 grams of water at...
Questions


Mathematics, 31.01.2020 10:43

History, 31.01.2020 10:43

Mathematics, 31.01.2020 10:43

Mathematics, 31.01.2020 10:43


Biology, 31.01.2020 10:43

English, 31.01.2020 10:43


Mathematics, 31.01.2020 10:43


Mathematics, 31.01.2020 10:43

History, 31.01.2020 10:43

Physics, 31.01.2020 10:43



Mathematics, 31.01.2020 10:43

Physics, 31.01.2020 10:43


Mathematics, 31.01.2020 10:43



