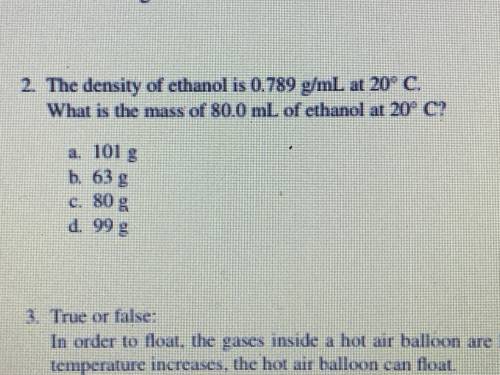
The density of ethanol is 0.789 g/mL at 20 c
what is the mass of 80.0 mL of ethanol at 2...

Chemistry, 13.01.2021 22:40 jakeyywashere
The density of ethanol is 0.789 g/mL at 20 c
what is the mass of 80.0 mL of ethanol at 20 c

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Questions


Advanced Placement (AP), 18.03.2021 01:50


Mathematics, 18.03.2021 01:50







Mathematics, 18.03.2021 01:50


History, 18.03.2021 01:50







Computers and Technology, 18.03.2021 01:50



