5 of 20
09
A chemist places zinc metal and hydrochloric acid in a test tube. She observes tha...

Chemistry, 14.01.2021 01:00 uchechukwueigwe
5 of 20
09
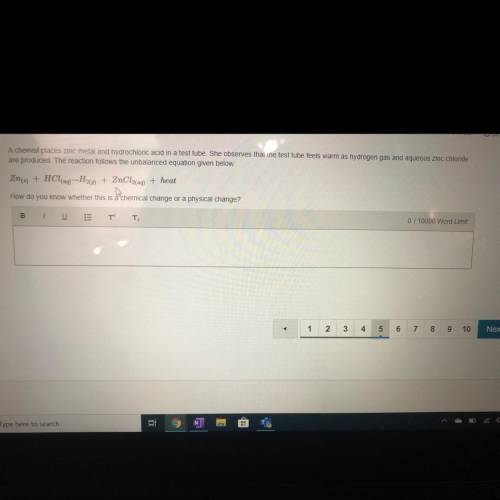
A chemist places zinc metal and hydrochloric acid in a test tube. She observes that the test tube feels warm as hydrogen gas and aqueous zinc chloride
are produced. The reaction follows the unbalanced equation given below.
Zn(s) + HCl(aq) - H20) + ZnCl2(aq) + heat
How do you know whether this is a chemical ct ge or a ysical change?
B
U
T
T:
0 / 10000 Word Limit

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Questions

Mathematics, 15.12.2021 20:10





Biology, 15.12.2021 20:10

English, 15.12.2021 20:10



English, 15.12.2021 20:10

Mathematics, 15.12.2021 20:10

Mathematics, 15.12.2021 20:10


Social Studies, 15.12.2021 20:10



Mathematics, 15.12.2021 20:10

Mathematics, 15.12.2021 20:10




