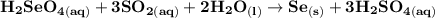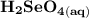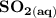Chemistry, 14.01.2021 16:40 angelreji386
Write a balanced equation describing the reduction of H2SeO4 by SO2 to produce selenium. (Use the lowest possible coefficients. Include states-of-matter under SATP conditions in your answer.)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

You know the right answer?
Write a balanced equation describing the reduction of H2SeO4 by SO2 to produce selenium. (Use the lo...
Questions


History, 02.08.2019 21:30

Spanish, 02.08.2019 21:30

Geography, 02.08.2019 21:30







Health, 02.08.2019 21:30






History, 02.08.2019 21:30

Business, 02.08.2019 21:30


Spanish, 02.08.2019 21:30