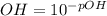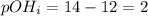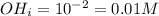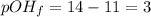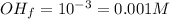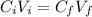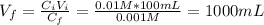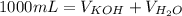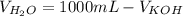Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
You are given 100 mL of of a KOH solution with pH of 12.0. You are required to change the pH to 11.0...
Questions

Social Studies, 28.08.2019 19:30

Health, 28.08.2019 19:30

English, 28.08.2019 19:30

Mathematics, 28.08.2019 19:30

History, 28.08.2019 19:30

Chemistry, 28.08.2019 19:30




Mathematics, 28.08.2019 19:30



Biology, 28.08.2019 19:30

History, 28.08.2019 19:30


Health, 28.08.2019 19:30

Chemistry, 28.08.2019 19:30

Computers and Technology, 28.08.2019 19:30

English, 28.08.2019 19:30

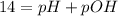
![pOH = -log[OH]](/tpl/images/1035/4467/6a17e.png)