
Chemistry, 14.01.2021 18:10 smallblueberry
i) Mass of CO2 formed = 2.241 g Molar mass of CO2 = Atomic mass of C + 2 (Atomic mass of O) = 12 + 2 (16) g/mol = 44 g/mol Thus, moles of carbon dioxide formed

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
i) Mass of CO2 formed = 2.241 g Molar mass of CO2 = Atomic mass of C + 2 (Atomic mass of O) = 12 + 2...
Questions

Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00


Biology, 15.04.2021 01:00

World Languages, 15.04.2021 01:00




Spanish, 15.04.2021 01:00








Mathematics, 15.04.2021 01:00


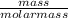
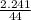 = 0.05mole
= 0.05mole

