
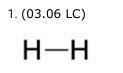
H-H Two side-by-side letter H's connected by a short, horizontal line segment. The Lewis structure for a hydrogen molecule, H2, is shown. The line segment in the structure shows that the two atoms (2 points) share one valence electron. share two valence electrons. have one unshared electron. have two unshared electrons.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1


Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
H-H Two side-by-side letter H's connected by a short, horizontal line segment. The Lewis structure f...
Questions

English, 04.11.2020 08:50

Mathematics, 04.11.2020 08:50

Mathematics, 04.11.2020 08:50




English, 04.11.2020 08:50



Chemistry, 04.11.2020 08:50

English, 04.11.2020 08:50



English, 04.11.2020 08:50


Mathematics, 04.11.2020 08:50






