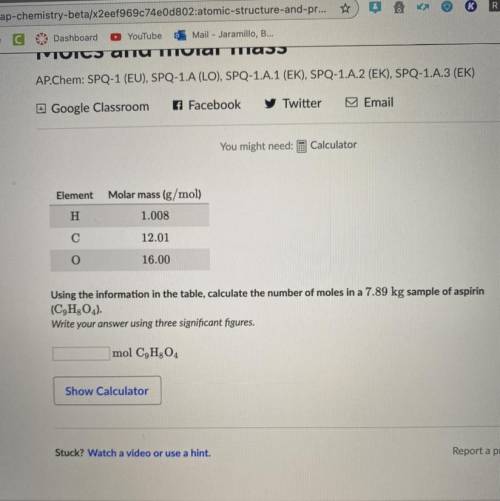
Element
Molar mass (g/mol)
H
1.008
С
12.01
O
16.00
Using...

Chemistry, 14.01.2021 19:10 FailingstudentXD
Element
Molar mass (g/mol)
H
1.008
С
12.01
O
16.00
Using the information in the table, calculate the number of moles in a 7.89 kg sample of aspirin (C9H2O4).
Write your answer using three significant figures.
mol C9H304
Show Calculator

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Questions


English, 17.05.2021 21:00

Geography, 17.05.2021 21:00

Mathematics, 17.05.2021 21:00



Mathematics, 17.05.2021 21:00

Spanish, 17.05.2021 21:00






English, 17.05.2021 21:00


Biology, 17.05.2021 21:00

English, 17.05.2021 21:00



History, 17.05.2021 21:00



