
Look at the following enthalpy diagram. Select all that apply.
A.)The products have more energy than the reactants.
B.)This is an addition reaction.
C.)A large activation energy is required for this reaction to take place.
D.)The products are more stable than the reactants.
E.)This is a substitution reaction.
You may have more than one answer.
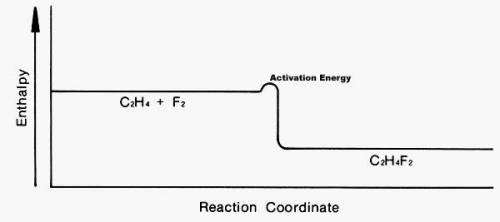

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Look at the following enthalpy diagram. Select all that apply.
A.)The products have more energy tha...
Questions

Biology, 28.08.2019 00:50


English, 28.08.2019 00:50

Mathematics, 28.08.2019 00:50


English, 28.08.2019 00:50

Biology, 28.08.2019 00:50



Mathematics, 28.08.2019 00:50

Mathematics, 28.08.2019 00:50

Physics, 28.08.2019 00:50

Mathematics, 28.08.2019 00:50

Biology, 28.08.2019 00:50









