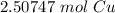Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
How many moles of copper are in 1.51 x 1024 Cu atoms?...
Questions














Mathematics, 12.11.2019 03:31






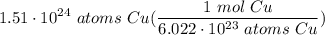 Multiply/Divide:
Multiply/Divide: