

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

You know the right answer?
4. Calculate the molar mass of Fe2(SO4)3. Then calculate the mass of iron(III) sulfate of 4.05X1023...
Questions

Mathematics, 18.09.2019 20:00

Social Studies, 18.09.2019 20:00




Mathematics, 18.09.2019 20:00

Health, 18.09.2019 20:00

History, 18.09.2019 20:00


Mathematics, 18.09.2019 20:00



Social Studies, 18.09.2019 20:00




Mathematics, 18.09.2019 20:00

Mathematics, 18.09.2019 20:00

Computers and Technology, 18.09.2019 20:00

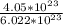 =269
=269

