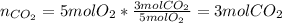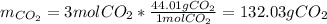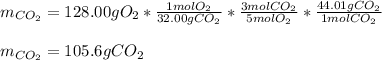A. Use the balanced equation C3H8 + 5O2 -> 3CO2 + 4H2O to answer the following questions
i. How many moles of CO2 are produced from 5 moles 02? (1 point)
ii. How many grams of CO2 are produced from 5 moles O2? (2 points)
iii how many grams of CO2 are produced from 128.00g O2? (2 points)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
A. Use the balanced equation C3H8 + 5O2 -> 3CO2 + 4H2O to answer the following questions
i. How...
Questions


Mathematics, 26.02.2021 14:20

Biology, 26.02.2021 14:20



Mathematics, 26.02.2021 14:20




Mathematics, 26.02.2021 14:20


English, 26.02.2021 14:20

English, 26.02.2021 14:20