
Chemistry, 15.01.2021 19:50 jrfranckowiak
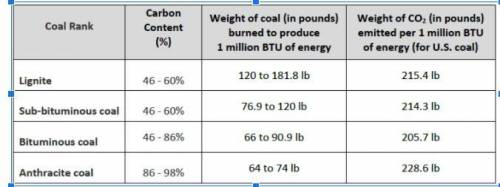
When coal is burned, carbon atoms are moved from the geosphere to the atmosphere. Each atom of carbon in the coal combines with two atoms of oxygen to produce carbon dioxide (CO2). Carbon dioxide has an atomic weight of 44, which is approximately 3.667 times heavier than a carbon atom of atomic weight 12. It takes about 4750 pounds of coal to power a house for one year. If anthracite coal is used, how many pounds of carbon will this be?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
When coal is burned, carbon atoms are moved from the geosphere to the atmosphere. Each atom of carbo...
Questions

Mathematics, 25.01.2022 14:00


Social Studies, 25.01.2022 14:00


English, 25.01.2022 14:00




Mathematics, 25.01.2022 14:00



Biology, 25.01.2022 14:00


Mathematics, 25.01.2022 14:00



English, 25.01.2022 14:00





