Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

You know the right answer?
Thermodynamics and Q
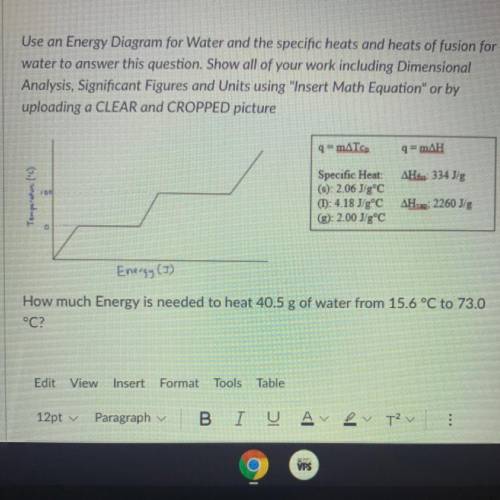
How much energy is needed to heat 40.5g of water from 15.6°C to 73.0°C <...
How much energy is needed to heat 40.5g of water from 15.6°C to 73.0°C <...
Questions


Mathematics, 28.09.2021 23:00


Mathematics, 28.09.2021 23:00

Mathematics, 28.09.2021 23:00

Mathematics, 28.09.2021 23:00


Advanced Placement (AP), 28.09.2021 23:00



Biology, 28.09.2021 23:00

Spanish, 28.09.2021 23:00

Chemistry, 28.09.2021 23:00


Mathematics, 28.09.2021 23:00

English, 28.09.2021 23:00

Mathematics, 28.09.2021 23:00

Mathematics, 28.09.2021 23:00

Arts, 28.09.2021 23:00