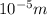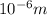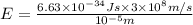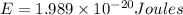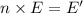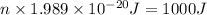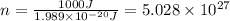Chemistry, 21.01.2020 11:31 bapehoodboi
Calculate thr number of photons having a wavelength of 10.0 μm required to produce 1.0 kj of energy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Calculate thr number of photons having a wavelength of 10.0 μm required to produce 1.0 kj of energy....
Questions

History, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

Social Studies, 07.01.2020 23:31


Mathematics, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

English, 07.01.2020 23:31


Mathematics, 07.01.2020 23:31

History, 07.01.2020 23:31






Mathematics, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

Arts, 07.01.2020 23:31

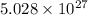 .
.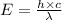
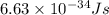
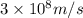
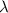 = wavelength = 10.0 μm =
= wavelength = 10.0 μm =