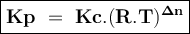Chemistry, 16.01.2021 14:00 savannah647
Kp/Kc for reaction for the equilibrium, A(g) ⇌ C(g)+B(g), is .
(Kc is the equilibrium constant in terms of concentrations, Kp is the equilibrium constant in terms of pressures, R is the gas constant, T is the temperature)
Select one:
(RT)2
(RT)-1
(RT)-2
(RT)-1.5
RT

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
Kp/Kc for reaction for the equilibrium, A(g) ⇌ C(g)+B(g), is .
(Kc is the equilibrium constant in t...
Questions

English, 21.01.2022 21:30


English, 21.01.2022 21:30


Mathematics, 21.01.2022 21:30

Mathematics, 21.01.2022 21:30

English, 21.01.2022 21:30

English, 21.01.2022 21:30

Mathematics, 21.01.2022 21:30

History, 21.01.2022 21:30

Mathematics, 21.01.2022 21:30



English, 21.01.2022 21:30

Advanced Placement (AP), 21.01.2022 21:30




Mathematics, 21.01.2022 21:40

![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/1041/5418/55d71.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/1041/5418/1041d.png)