
Chemistry, 16.01.2021 14:40 jakeyywashere
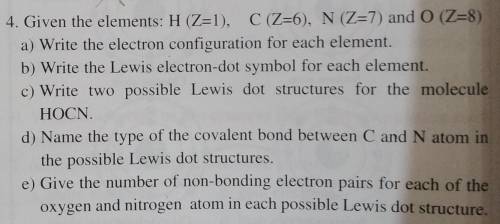
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration for each element.
b) Write the Lewis electron-dot symbol for each element.
c) Write two possible Lewis dot structures for the molecule
HOCN.
d) Name the type of the covalent bond between C and N atom in
the possible Lewis dot structures.
e) Give the number of non-bonding electron pairs for each of the
oxygen and nitrogen atom in each possible Lewis dot structure

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1


Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1
You know the right answer?
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration fo...
Questions











History, 30.06.2019 03:00









History, 30.06.2019 03:00



