Chemistry, 16.01.2021 23:50 robertjoy19
A sample of carbon dioxide gas at a pressure of 891 mm Hg and a temperature of 84 C occupies a volume of 5.61 L. If the gas is cooled at constant pressure to a temperature of 40 C, the volume of the gas will be __ L.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

You know the right answer?
A sample of carbon dioxide gas at a pressure of 891 mm Hg and a temperature of 84 C occupies a volum...
Questions



History, 29.10.2020 04:10


World Languages, 29.10.2020 04:10

Law, 29.10.2020 04:10

Mathematics, 29.10.2020 04:10

English, 29.10.2020 04:10



Mathematics, 29.10.2020 04:10

Mathematics, 29.10.2020 04:10


History, 29.10.2020 04:10


Mathematics, 29.10.2020 04:10

History, 29.10.2020 04:10



History, 29.10.2020 04:10

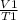 =
= 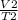
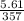 =
=