
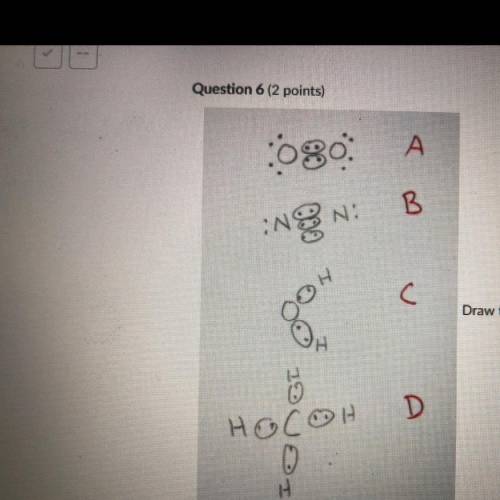
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select
the answer that best describes which drawing is wrong and why. Note I drew circles
around electrons that are participating in covalent bonding. This is normally not done
but for the purpose of this test the circled electrons are fine.
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the
Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions

Mathematics, 29.08.2019 22:00

History, 29.08.2019 22:00

Chemistry, 29.08.2019 22:00

Advanced Placement (AP), 29.08.2019 22:00



Health, 29.08.2019 22:00

Social Studies, 29.08.2019 22:00

Computers and Technology, 29.08.2019 22:00

Mathematics, 29.08.2019 22:00


Mathematics, 29.08.2019 22:00




Biology, 29.08.2019 22:00


English, 29.08.2019 22:00


Geography, 29.08.2019 22:00



