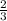Chemistry, 17.01.2021 01:10 amiahmiller79
Given the unbalanced equation: CaSO4 + ___AlCl3 --> Al2(SO4)3 + CaCl2 What is the coefficient of Al2(SO4)3 when the equation is completely balanced using the smallest whole-number coefficients?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
Given the unbalanced equation: CaSO4 + ___AlCl3 --> Al2(SO4)3 + CaCl2
What is the coefficient...
Questions


Law, 05.02.2021 01:50




English, 05.02.2021 01:50

Mathematics, 05.02.2021 01:50

Social Studies, 05.02.2021 01:50


Mathematics, 05.02.2021 01:50



Mathematics, 05.02.2021 01:50

Biology, 05.02.2021 01:50



Mathematics, 05.02.2021 01:50

Mathematics, 05.02.2021 01:50


History, 05.02.2021 01:50

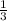 , b =
, b =