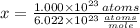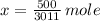Estimate: Press Reset. Select Atoms, and using the slider, start with 1.000 × 1023 atoms of sulfur. (Note that pressing Start puts atoms into the atom counter, not the jars.)
Is this amount more or less than one mole?
Place the jar underneath the counter. Was the jar completely filled?
How many moles do you have?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Estimate: Press Reset. Select Atoms, and using the slider, start with 1.000 × 1023 atoms of sulfur....
Questions






English, 09.10.2019 16:30





Geography, 09.10.2019 16:30

Mathematics, 09.10.2019 16:30


English, 09.10.2019 16:30


Mathematics, 09.10.2019 16:30




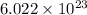 atoms. Given that we have
atoms. Given that we have 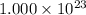 atoms, then we conclude that this amount is less than one mole.
atoms, then we conclude that this amount is less than one mole.