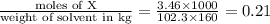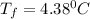Chemistry, 17.01.2021 16:10 rogersdeloris1ovgm3b
calculate the freezing point of 3.46 gram of a compound X in 160 gram of benzene when a separate sample of X was vaporized it's density was found to be 3.27 gram/liter at 116°c and 773 torr. The freezing point of pure benzene is 5.45°c of Kf=5.1°/m

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1


You know the right answer?
calculate the freezing point of 3.46 gram of a compound X in 160 gram of benzene when a separate sam...
Questions


Mathematics, 11.03.2021 20:20


Mathematics, 11.03.2021 20:20


Mathematics, 11.03.2021 20:20

Computers and Technology, 11.03.2021 20:20


Mathematics, 11.03.2021 20:20



Mathematics, 11.03.2021 20:20

Mathematics, 11.03.2021 20:20


Social Studies, 11.03.2021 20:20


History, 11.03.2021 20:20

Social Studies, 11.03.2021 20:20

Mathematics, 11.03.2021 20:20

Mathematics, 11.03.2021 20:20

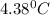

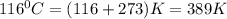
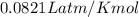
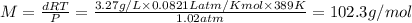
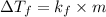
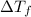 = depression in freezing point =
= depression in freezing point = 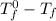 =
= 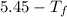
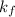 = freezing point constant = 5.1
= freezing point constant = 5.1