Chemistry, 17.01.2021 18:40 sierraaasifuent
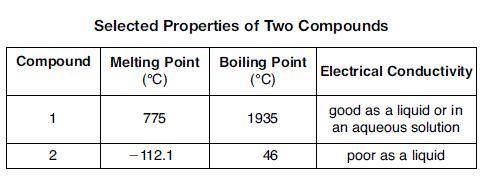
The table below shows properties of two compounds at standard pressure. Which statement classifies the two compounds? 1)Both compounds are ionic, 2)Both compounds are molecular, 3) Compound 1 is ionic, and compound 2 is molecular, 4)Compound 1 is molecular, and compound is ionic

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
The table below shows properties of two compounds at standard pressure. Which statement classifies t...
Questions


Mathematics, 09.04.2020 22:08



Mathematics, 09.04.2020 22:08

Biology, 09.04.2020 22:08


Business, 09.04.2020 22:08

Mathematics, 09.04.2020 22:08



Mathematics, 09.04.2020 22:09

Social Studies, 09.04.2020 22:09

History, 09.04.2020 22:09


History, 09.04.2020 22:09


Mathematics, 09.04.2020 22:09


Spanish, 09.04.2020 22:09