
Chemistry, 18.01.2021 07:00 Jenniferwolf
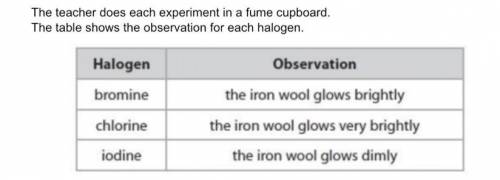
A student states that the order of reactivity cannot be found from this experiment because bromine is a liquid, chlorine is a gas and iodine is a solid at room temperature. Evaluate the student's statement.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

You know the right answer?
A student states that the order of reactivity cannot be found from this experiment because bromine i...
Questions

Mathematics, 13.04.2020 22:00

Mathematics, 13.04.2020 22:00


Biology, 13.04.2020 22:00



Geography, 13.04.2020 22:00

Mathematics, 13.04.2020 22:00



English, 13.04.2020 22:00





History, 13.04.2020 22:00


Computers and Technology, 13.04.2020 22:00




