(3)
Calculate the entropy change when a sample of argon at 25 °C and 1.00 atm in a container
o...

Chemistry, 18.01.2021 14:00 superbatman9193
(3)
Calculate the entropy change when a sample of argon at 25 °C and 1.00 atm in a container
of volume 500 cm3 is compressed to 50 cm3 and cooled to -25 °C.
For argon, Cpm = 20.786) K-Imol-1. Assume that argon behaves perfectly.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
Questions

Mathematics, 14.08.2020 18:01



Social Studies, 14.08.2020 18:01


Mathematics, 14.08.2020 18:01







Mathematics, 14.08.2020 18:01

Chemistry, 14.08.2020 18:01

History, 14.08.2020 18:01

Mathematics, 14.08.2020 18:01




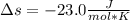
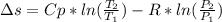
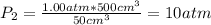
![\Delta s =20.786\frac{J}{mol*K} *ln[\frac{(-25+273)K}{(25+273)K} ]-8.3145\frac{J}{mol*K}*ln(\frac{10atm}{1atm} )\\\\\Delta s=-23.0\frac{J}{mol*K}](/tpl/images/1043/0995/45d4a.png)


