
Chemistry, 18.01.2021 14:00 haileyglowiak8183
Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HNO3 (aq)+ H2O â

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Predict the products of the reaction below. That is, complete the right-hand side of the chemical eq...
Questions

Mathematics, 07.10.2020 08:01

Mathematics, 07.10.2020 08:01

English, 07.10.2020 08:01


History, 07.10.2020 08:01


English, 07.10.2020 08:01





English, 07.10.2020 08:01



Mathematics, 07.10.2020 08:01

Mathematics, 07.10.2020 08:01

Mathematics, 07.10.2020 08:01

Mathematics, 07.10.2020 08:01


Arts, 07.10.2020 08:01

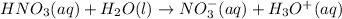
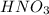 is a stronger acid than water , it will lose
is a stronger acid than water , it will lose 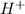 ions and water will gain
ions and water will gain 

