
Chemistry, 18.01.2021 18:10 mallorytaylor279
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydroxide solution of concentration 0.15 mol/dm3.
The equation which represents the reaction is:
HCl + NaOH > NaCl + H2O
Calculate the concentration of hydrochloric acid in mol/dm3

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement accurately describes the rock layers? layer 8 is older than layer 1. layer 3 is younger than layer 6. layer 4 and layer 10 are the same relative age. layer 2 and layer 9 are the same relative age.
Answers: 3

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydr...
Questions


Computers and Technology, 14.02.2020 17:10


Mathematics, 14.02.2020 17:10


Chemistry, 14.02.2020 17:11



Health, 14.02.2020 17:11



English, 14.02.2020 17:11








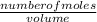 =
= 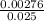 = 0.11mol/dm³
= 0.11mol/dm³


