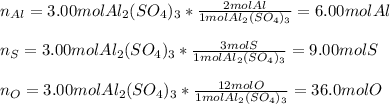Chemistry, 18.01.2021 21:10 jiiaxuan2035
Calculate the number of moles of aluminum, sulfur, and oxygen atoms in 3.00 molesmoles of aluminum sulfate, Al2(SO4)3Al2(SO4)3. Express the number of moles of AlAl, SS, and OO atoms numerically, separated by commas.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Calculate the number of moles of aluminum, sulfur, and oxygen atoms in 3.00 molesmoles of aluminum s...
Questions

History, 14.08.2019 08:30

Mathematics, 14.08.2019 08:30


Biology, 14.08.2019 08:30





Mathematics, 14.08.2019 08:30




Mathematics, 14.08.2019 08:30




Mathematics, 14.08.2019 08:30