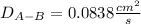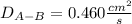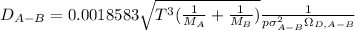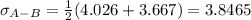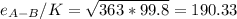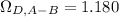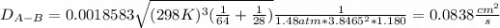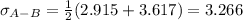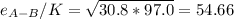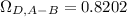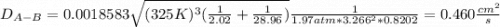Chemistry, 18.01.2021 21:10 KittyLoverCat
Estimate the value of the gas-phase diffusion coefficient for the following pairs using the Hirshfelder equation: a. Sulfur dioxide and nitrogen (N2) at 298 K and 1.5 x 105 Pa b. Hydrogen (H2) and air at 325 K and 2.0 x 105 Pa

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Estimate the value of the gas-phase diffusion coefficient for the following pairs using the Hirshfel...
Questions

Mathematics, 15.07.2020 03:01

History, 15.07.2020 03:01





Mathematics, 15.07.2020 03:01



Mathematics, 15.07.2020 03:01