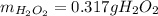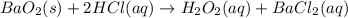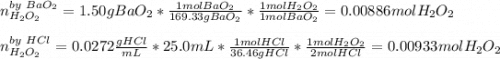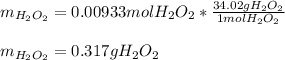Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
What amount of hydrogen peroxide should result ( theoretical yield) when 1.50g of barium peroxide is...
Questions

Mathematics, 18.11.2019 23:31

Mathematics, 18.11.2019 23:31




History, 18.11.2019 23:31

History, 18.11.2019 23:31

History, 18.11.2019 23:31




History, 18.11.2019 23:31


English, 18.11.2019 23:31



Health, 18.11.2019 23:31

Chemistry, 18.11.2019 23:31


Biology, 18.11.2019 23:31