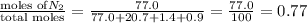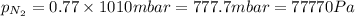Chemistry, 18.01.2021 21:10 jones501324
1. Consider the following properties of the atmosphere near the coast in Southern California: Average surface pressure: 1010 mbar Average temperature: 295 K Atmospheric gas composition (by volume): Nitrogen (N2) 77.0%, Oxygen (O2) 20.7%, Water (H2O) 1.4%, Argon (Ar), 0.9%. a. What is the average partial pressure of N2 in the atmosphere, pA, in units of Pa

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
1. Consider the following properties of the atmosphere near the coast in Southern California: Averag...
Questions

Mathematics, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00


History, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00



Mathematics, 29.10.2021 14:00



English, 29.10.2021 14:00

History, 29.10.2021 14:00




Geography, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00



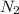 in the atmosphere is 77770 Pa.
in the atmosphere is 77770 Pa.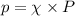
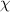 = mole fraction
= mole fraction 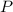 = total pressure
= total pressure