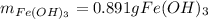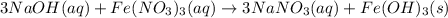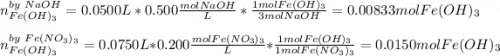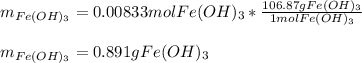Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
How many grams of iron(III) hydroxide (106.87 g/mol) will precipitate if 50.0 mL of 0.500 M sodium h...
Questions




Mathematics, 12.11.2019 02:31

Mathematics, 12.11.2019 02:31

Physics, 12.11.2019 02:31


Mathematics, 12.11.2019 02:31

Mathematics, 12.11.2019 02:31




English, 12.11.2019 02:31



Health, 12.11.2019 02:31


Mathematics, 12.11.2019 02:31