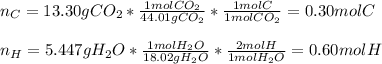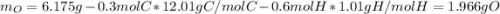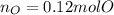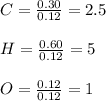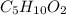Chemistry, 18.01.2021 21:40 5924000264
A 6.175 gram sample of an organic compound containing only C, H, and O is analyzed by combustion analysis and 13.30 g CO2 and 5.447 g H2O are produced. In a separate experiment, the molar mass is found to be 102.1 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3


Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3
You know the right answer?
A 6.175 gram sample of an organic compound containing only C, H, and O is analyzed by combustion ana...
Questions



Mathematics, 06.05.2020 20:05


Mathematics, 06.05.2020 20:05

Physics, 06.05.2020 20:05

Mathematics, 06.05.2020 20:05

Mathematics, 06.05.2020 20:05


Mathematics, 06.05.2020 20:05

Mathematics, 06.05.2020 20:05


History, 06.05.2020 20:05

English, 06.05.2020 20:05


Social Studies, 06.05.2020 20:05