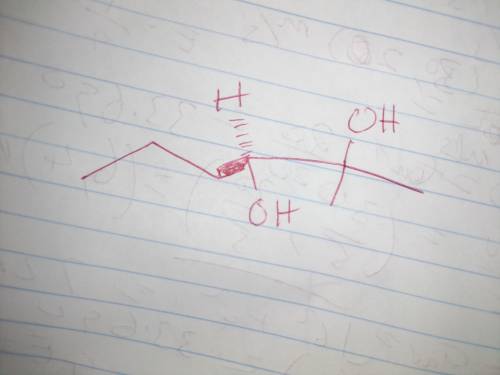
Chemistry, 19.01.2021 02:00 nschavez123
Draw the major product formed when the given epoxide reacts with aqueous acid. Use wedge and dash bonds, including hydrogen atoms at each stereogenic center, to show the stereochemistry of the product.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Draw the major product formed when the given epoxide reacts with aqueous acid. Use wedge and dash bo...
Questions




Mathematics, 04.03.2021 20:20



English, 04.03.2021 20:20

English, 04.03.2021 20:20


Biology, 04.03.2021 20:20


Advanced Placement (AP), 04.03.2021 20:20

English, 04.03.2021 20:20


Chemistry, 04.03.2021 20:20

Computers and Technology, 04.03.2021 20:20

Advanced Placement (AP), 04.03.2021 20:20

Mathematics, 04.03.2021 20:20


SAT, 04.03.2021 20:20