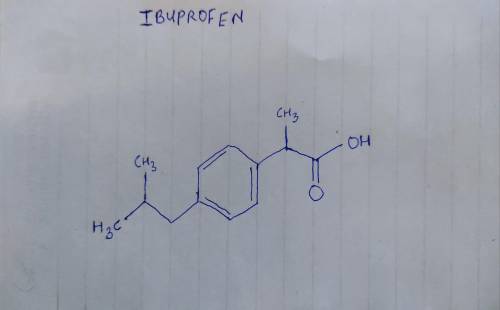
Ibuprofen (aka ADVIL) is a weak acid with a pKa of 4.9. It is absorbed through the stomach and the small intestine as a function of polarity - charged and very polar molecules are absorbed slowly; neutral hydrophobic molecules absorb quickly. If the stomach pH is about 1.5 and the small intestine pH is about 6, where (and why) will more ibuprofen be absorbed into the bloodstream

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Ibuprofen (aka ADVIL) is a weak acid with a pKa of 4.9. It is absorbed through the stomach and the s...
Questions

Biology, 08.07.2019 02:00


Mathematics, 08.07.2019 02:00


History, 08.07.2019 02:00

History, 08.07.2019 02:00

History, 08.07.2019 02:00

History, 08.07.2019 02:00


Mathematics, 08.07.2019 02:00

Mathematics, 08.07.2019 02:00


Mathematics, 08.07.2019 02:00

Mathematics, 08.07.2019 02:00

Mathematics, 08.07.2019 02:00


History, 08.07.2019 02:00

Mathematics, 08.07.2019 02:00

Biology, 08.07.2019 02:00