
In an experiment, potassium chlorate decomposed according to the following chemical equation.
KClO3 → KCl + O2
(Molar mass of KClO3 = 122.5 g/mol; KCl = 74.55 g/mol; O2 = 31.998 g/mol)
If the mass of potassium chlorate was 240 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?
(240 × 2 × 31.998) ÷ (122.5 × 3) grams
(240 × 3 × 31.998) ÷ (122.5 × 2) grams
(240 × 2 × 122.5) ÷ (31.998 × 3) grams
(240 × 3 × 122.5) ÷ (31.998 × 2) grams

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2


You know the right answer?
In an experiment, potassium chlorate decomposed according to the following chemical equation.
KClO3...
Questions



Mathematics, 04.01.2021 21:40

Mathematics, 04.01.2021 21:40

English, 04.01.2021 21:40


English, 04.01.2021 21:40




History, 04.01.2021 21:40


Mathematics, 04.01.2021 21:40



Mathematics, 04.01.2021 21:40



Mathematics, 04.01.2021 21:40

World Languages, 04.01.2021 21:40

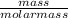
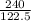
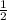 x 31.998
x 31.998

