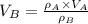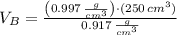Chemistry, 19.01.2021 14:00 Jazongamez1987
At 25.0 °C the density of liquid water is 0.997 g/cm3, but at -10.0 °C the density of solid water (ice) is 0.917 g/cm3. If a 250.0 mL sample of liquid water originally at 25.0 °C is frozen and cooled to -10.0 °C, what volume will the solid occupy?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2


Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2

You know the right answer?
At 25.0 °C the density of liquid water is 0.997 g/cm3, but at -10.0 °C the density of solid water (i...
Questions



Mathematics, 14.11.2019 06:31

Mathematics, 14.11.2019 06:31


Mathematics, 14.11.2019 06:31




Mathematics, 14.11.2019 06:31


History, 14.11.2019 06:31



Geography, 14.11.2019 06:31

English, 14.11.2019 06:31


History, 14.11.2019 06:31

Social Studies, 14.11.2019 06:31

 (1)
(1)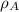 ,
,  - Densities of water at 25 ºC and - 10 ºC, measured in grams per cubic centimeter.
- Densities of water at 25 ºC and - 10 ºC, measured in grams per cubic centimeter.  ,
, 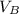 - Volume occupied by the water at 25 ºC and - 10 ºC, measured in cubic centimeters.
- Volume occupied by the water at 25 ºC and - 10 ºC, measured in cubic centimeters.  ,
,  and
and 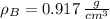 , then the volume occupied by the water at - 10 ºC is:
, then the volume occupied by the water at - 10 ºC is: