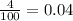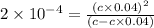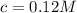Chemistry, 19.01.2021 14:00 jenisimonsow6ddf
Calculate molar concentration of formic acid solution if degree of dissociation is 4% K(HCOOH)=2.10^_4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3
You know the right answer?
Calculate molar concentration of formic acid solution if degree of dissociation is 4% K(HCOOH)=2.10^...
Questions

English, 04.02.2021 06:00

Mathematics, 04.02.2021 06:00

Mathematics, 04.02.2021 06:00

History, 04.02.2021 06:00

English, 04.02.2021 06:00


History, 04.02.2021 06:00




Mathematics, 04.02.2021 06:00




Mathematics, 04.02.2021 06:00



Mathematics, 04.02.2021 06:00

Mathematics, 04.02.2021 06:00

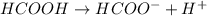
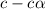
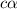
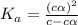
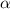 =
=