
Chemistry, 19.01.2021 14:00 ariana7245
10.Calculate the volume of oxygen that reacts with 500cm3 of hydrogen sulfide with the volume of both gases measured at rtp: 2H2S + 3O2 > 2SO2 + 2H20

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
10.Calculate the volume of oxygen that reacts with 500cm3 of hydrogen sulfide with the volume of bot...
Questions


Mathematics, 09.01.2021 01:10

History, 09.01.2021 01:10

Mathematics, 09.01.2021 01:10


Mathematics, 09.01.2021 01:10

Mathematics, 09.01.2021 01:10



Social Studies, 09.01.2021 01:10

English, 09.01.2021 01:10



English, 09.01.2021 01:10

History, 09.01.2021 01:10




Mathematics, 09.01.2021 01:10

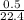 = 0.022mole of hydrogen sulfide;
= 0.022mole of hydrogen sulfide;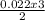 = 0.033mole of O₂
= 0.033mole of O₂

