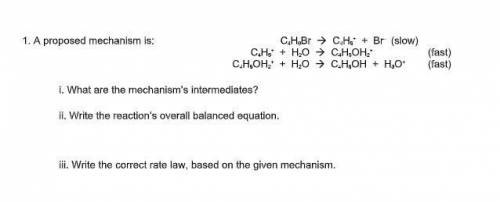
A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (f...

A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (fast)
C4H9OH2^+ + H2O --> C4H9OH + H3O^+ (fast)
i. What are the mechanism’s intermediates?
ii. Write the reaction’s overall balanced equation.
iii. Write the correct rate law, based on the given mechanism.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

You know the right answer?
Questions




Mathematics, 11.03.2021 22:50

Mathematics, 11.03.2021 22:50







Mathematics, 11.03.2021 22:50

Mathematics, 11.03.2021 22:50


History, 11.03.2021 22:50

Mathematics, 11.03.2021 22:50

Mathematics, 11.03.2021 22:50

Mathematics, 11.03.2021 22:50

Mathematics, 11.03.2021 22:50



