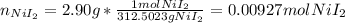Suppose 2.90g of nickel(II) iodide is dissolved in 150ml of a 0.70M aqueous solution of potassium carbonate. Calculate the final molarity of iodide anion in the solution. You can assume the volume of the solution doesn't change when the nickel(II) iodide is dissolved in it. Round your answer to 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Suppose 2.90g of nickel(II) iodide is dissolved in 150ml of a 0.70M aqueous solution of potassium ca...
Questions


English, 15.12.2020 23:30




Social Studies, 15.12.2020 23:30

Mathematics, 15.12.2020 23:30


Mathematics, 15.12.2020 23:30





English, 15.12.2020 23:30

Mathematics, 15.12.2020 23:30

Mathematics, 15.12.2020 23:30




Mathematics, 15.12.2020 23:30