Chemistry, 19.01.2021 19:10 Bangggggg6
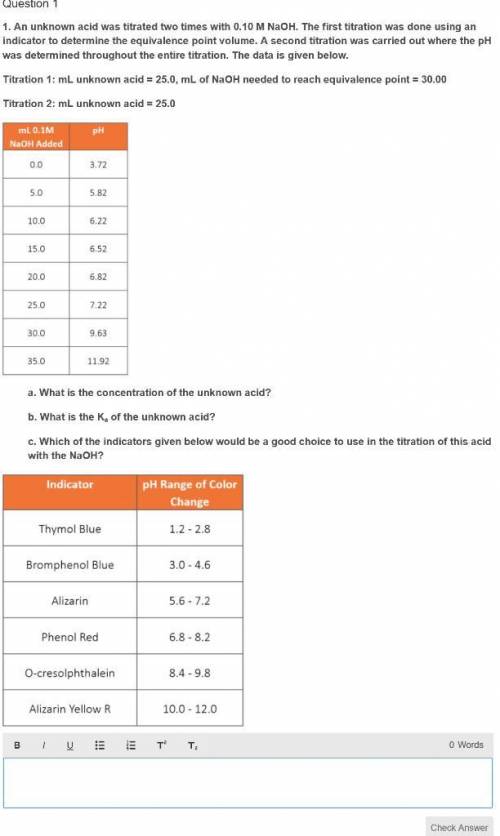
An unknown acid was titrated two times with 0.10 M NaOH. The first titration was done using an indicator to determine the equivalence point volume. A second titration was carried out where the pH was determined throughout the entire titration. The data is given below.
Titration 1: mL unknown acid = 25.0, mL of NaOH needed to reach equivalence point = 30.00
Titration 2: mL unknown acid = 25.0
mL 0.1M pH
NaOH Added
0.0 3.72
5.0 5.82
10.0 6.22
15.0 6.52
20.0 6.82
25.0 7.22
30.0 9.63
35.0 11.92
a. What is the concentration of the unknown acid?
b. What is the Ka of the unknown acid?
c. Which of the indicators given below would be a good choice to use in the titration of this acid with the NaOH?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
An unknown acid was titrated two times with 0.10 M NaOH. The first titration was done using an indic...
Questions


English, 27.05.2020 22:04


Biology, 27.05.2020 22:04


Mathematics, 27.05.2020 22:04



Mathematics, 27.05.2020 22:04


Mathematics, 27.05.2020 22:04









Engineering, 27.05.2020 22:04