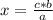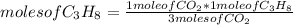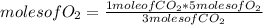Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2
You know the right answer?
C3H8 (g) 5O2 (g) -> 3CO2 (g) 4H2O (l) in the reaction represented baove, what is the total number...
Questions



Mathematics, 03.07.2021 03:30

Mathematics, 03.07.2021 03:30


Mathematics, 03.07.2021 03:30


Business, 03.07.2021 03:30









Mathematics, 03.07.2021 03:30

Chemistry, 03.07.2021 03:30


Mathematics, 03.07.2021 03:30